Hydrogenated Styrenic Thermoplastic Eastomer (SEBS) Tuftec™ H1221
Applications
Physical Form
- Supplied as a dusted, dence pellet 2 types of dusting agents
Features and Benefits
- Substitute for PVC
- Excellent flexibility
- Excellent compatibility to PP
- Good transparency
- Strong adhesion property
- Strong heat seal property
- Medical use
Regulations
- FDA 21 CFR FCN821
- Regulation (EU) No 10/2011
- USP Class Ⅵ
- Drug master File registered
Characteristics
| Property | Test Method | Unit | Value |
|---|---|---|---|
| Density | ISO 1183 | g/cm3 | 0.89 |
| MFR 230°C, 2.16kg Load | ISO 1133 | g/10 min | 4.5 |
| Hardness Durometer Type A |
ISO 7619 | °Shore A | 42 |
| Tensile Strength Dumbbell: Type 1A 500 mm/min |
ISO 37 | MPa | 9.5 |
| Elongation Dumbbell: Type 1A 500 mm/min |
ISO 37 | % | 980 |
| 300% Tensile Stress | ISO 37 | MPa | 1.0 |
| Styrene / Ethylene-Butylene Ratio | Asahi Kasei Method | – | 12/88 |
Please note that all data and values are given as typical results obtained with the indicated test methods for purposes of basic reference in grade selection only, and not as any product specification or warranty of any nature, and are subject to change without notice.
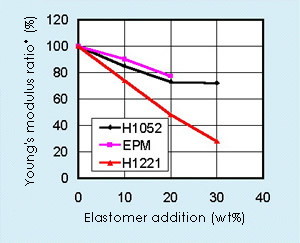
The flexibility of polypropylene is improved substantially by adding Tuftec™ H1221. The transparency and flexibility can be obtained by selecting appropriate polypropylene.
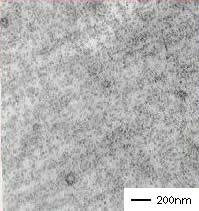
Tuftec™ H1221 shows nanometer-order dispersion in PP, imparting excellent flexibility to the production of polypropylene moldings.
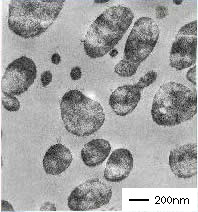
Typical characteristics of PP/H1221, in comparison with PP/H1052*
| Molding Method | Property | Test Method | SEBS H1221 |
SEBS H1052 |
|---|---|---|---|---|
| Extruded Sheet | Young’s Modulus (MPa) MD/TD |
ISO 527-3 | 340/380 | 700/550 |
| Tensile Yield Strength (MPa) MD/TD |
5/5 | 7/6 | ||
| Tensile Strength (MPa) MD/TD |
13/13 | 18/18 | ||
| Elongation (%) MD/TD |
70/71 | 72/66 | ||
| Total Light Ttransmission (%) 70μm Thick |
JIS K 7105 | 92.4 | 91.5 | |
| Haze (%) 70μm Thick |
4.2 | 17.8 | ||
| Whitening (%) ΔT |
Asahi Kasei Method | 3.2 | 33.3 | |
| Injection Sheet | Flexible Modulus (MPa)
2 mm/min Flexure |
ISO527-1 ISO 527-2 |
710 | 1,100 |
| Tensile Yield Strength (MPa) 50 mm/min Tension |
23 | 29 | ||
| Tensile Strength (MPa) 50 mm/min Tension |
28 | 21 | ||
| Elongation (%) 50 mm/min Tension |
530 | 730 | ||
| Brittle Temperature °C | ASTM D 746 | -21.4 | <-30 |
*Ratio: h-PP/Tuftec™ = 80/20
<Test Conditions>
Young’s modulus: Test piece 20 mm x 100 mm x 70 µm, rate of tension 2 mm/min
Whitening: Difference of total light transmission before and after DuPont impact test
Test piece 0.4 mm sheet; missile 1/2 in., 1 kg, 50 cm drop height
Please note that all data and values are given as typical results obtained with the indicated test methods for purposes of basic reference in grade selection only, and not as any product specification or warranty of any nature, and are subject to change without notice.
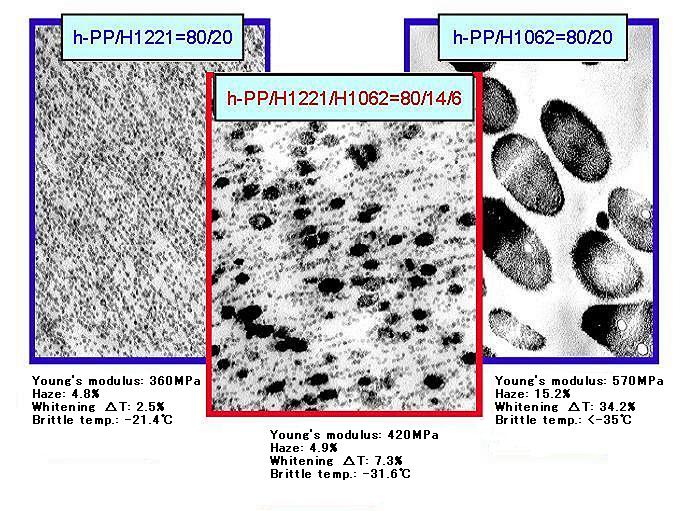
By blending Tuftec™ H1221 and H1062, transparent PP compound with excellent low-temperature brittleness resistance can be obtained.
Tuftec™ H1221 shows strong adhesion property to PMMA plate, and can be co-extruded with PP.

<Evaluation Process>
Adherend: PET film with 50µm thickness
Adhesive layer: Approx. 10µm thickness (Dried after solution coating)
Adhesion temp.:180 deg. C, Peeling temp.: 23 deg. C
Sample: 20mm width, Peeling speed: 300mm/min
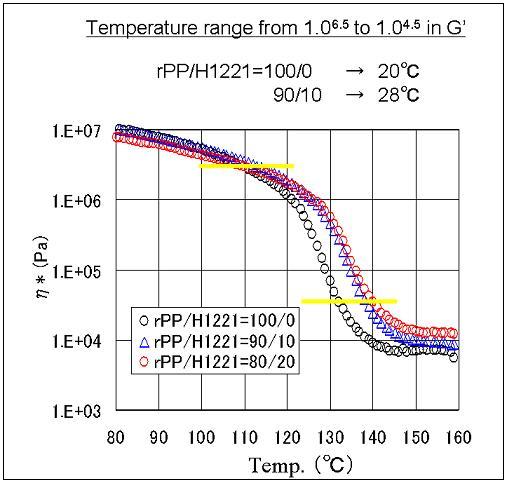
The viscosity change of PP can be moderated by adding Tuftec™ H1221
Please refer to Terms and Conditions and Privacy Policy
IMPORTANT NOTICE REGARDING MEDICAL APPLICATIONS
We, Asahi Kasei Group, request that customers who are considering using our products in medical, pharmaceutical, cosmetic and other related applications (hereinafter collectively called “Medical Applications”) shall contact us and confirm our policy on Medical Applications in advance. Information herein regarding conformity to certain laws and regulations are as of January, 2015. Please consult with us for the latest status. We make no guarantees or warranties, express or implied, concerning the suitability of our products for use in Medical Applications. It is not our responsibility to determine if our products are safe, lawful, and technically suitable for intended applications.